After trying my best to persuade an individual that dish soap does not strip wax, to no avail, I find myself compelled to provide some useful information regarding stripping wax. This is a bit of a vent about the wive's tales and the way information has been twisted over the years. Keep in mind that there is always some truth to these myths, but, as is commonly the case, the nature of the wive's tale is far from applicable to the current state of affairs.
This particular tale revolves around using dish soap to remove wax. There are several theories that promote this opinion. Below, we will explore these theories in detail and try to dispel the myth.
The first observation is that after using dish soap, the beading is gone, which indicates that the protection has been removed. I think you'll find that even when used properly for washing dishes that water lies flat on the dish and sheets off. This is a charactaristic that has been thoughtfully crafted into your dish soap. It's advantageous to have water sheet of your dishes to prevent waterspots. This is achieved through the use of surfactants. These surfactants act the same way on your car's paint. They sit on the surface and inhibit beading, but promote a sheeting action. Since the wax that was on your paint beaded water, one may conclude that the wax is gone becuase of the change in the surface properties. However, with the application of a mild solvent, such as isopropyl alcohol, one can strip the surfactants and find that the beading and wax still remain.
The next theory is that the pH of the dish soap is much higher than the wax and this degrades the wax. This may have been the case 30-50 years ago when dish soap contained strong alkaline constituents for cleaning purposes, but current dish soaps are near neutral in pH, if only slightly above pH 7 and as such, this is just plain wrong. Moreover, plenty of bench and field testing has been done with modern protectants that shows high pH products such as APC, do not in fact remove the protectant.
The next argument is that dish soaps are more acidic that waxes and therefore remove them. A simple check of the pH of common dish soaps will find that they are slightly above pH 7 and wax is typically around pH 8. While the dish soap has a lower pH, is it not more acidic. Since it is still above pH 7, it is merely less alkaline. This is an important distinction in that, as above, even strong alkalines do not strip the wax. If the pH is above 7, then the argument of it being more acidic is moot as it isn't at all acidic.
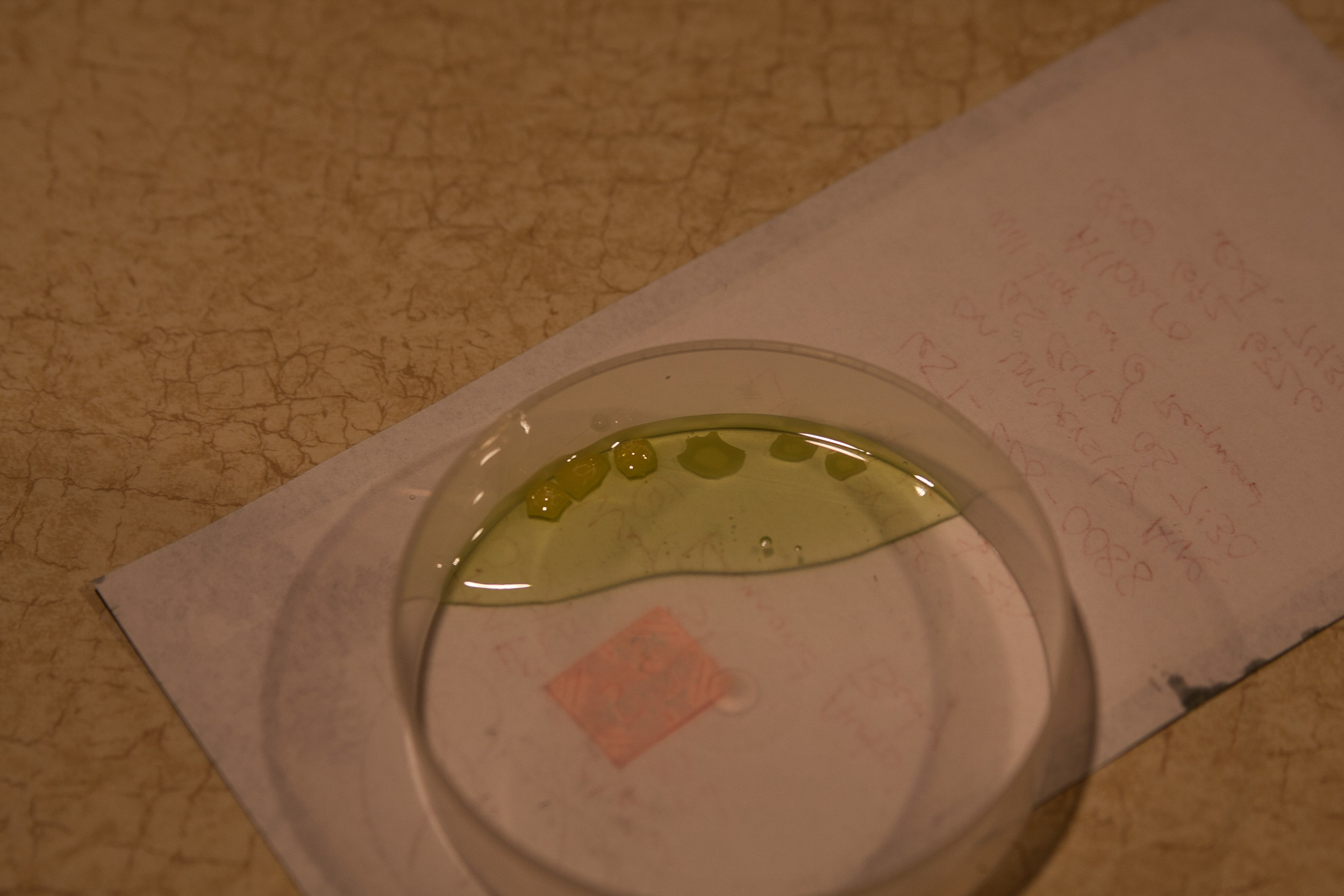
So to put the wive's tales of dish soap to bed, I've placed both beeswax and carnuaba wax in a container with dish soap where it has undergone continuous exposure for 12 months. The results? See for yourself. No visible change to the beeswax or the carnuaba wax. If spending a year in dish soap hasn't broken down these common wax constituents, than good luck doing it in 30 minutes.
Dishsoap upon application
Dishsoap 1 year later
The next argument is that solvents will remove waxes. This has a bit more merit, afterall, there are solvents in paste wax to make it more malleable. And there are plenty of solvents sold as 'wax and grease removers'. But, those who have made a paste wax know that there are certain steps taken, like heating the solvent and the wax to obtain a smooth consistency. Part of this is because of the much high ratio of wax to solvent; but the question is, at a much lower ratio, will the wax dissolve? Again, see for yourself how they performed during continuous exposure to naptha over the course of 12 months. The beeswax started to dissolve in 15 minutes. By 3 hours there was not much left and at 30 hours, it was gone. However, the carnuaba exhibited no change over the course of a year.
Wax upon application, beeswax already reacting
3 hours later, beeswax is dissolving, no reaction for carnuaba
1 year later, carnauba still hanging in there
For the conclusions of this exercise, we will assume a very basic wax formulation of solvent, carrier (heavy distillates) and waxes (beeswax and carnuaba). It should be noted that you may also find other additives like cetyl esters and siloxanes in a normal wax formulation. We can expect that the solvents will evaporate very quickly upon initial application of wax and the carriers will evaporate relatively slowly, but one could expect them to be gone by the time we try our first wash (perhaps a week after application). So what we are left with is beeswax and carnuaba wax forming a mechanical bond (entanglement) to the vehicles surface.
When we apply a dish soap, we can expect no chemically related degradation of the beeswax or carbuaba. But we can expect that the surfactants will modify the surface properties to make water lay flat and give the appearance that the wax is gone. But removing the surfactants will result in the surface properties being miraculously restored.
When we apply a solvent, we can expect some of the beeswax to be dissolved and if left long enough, perhaps all of it, but the carnuaba will be unaffected. This suggests that while solvents will degrade wax, there is a limit to the degradation. Once all the beeswax is gone, then you are left with the carnuaba and solvent initiated degradation would cease.
So if the chemicals discussed about won't remove wax, what if we use a mechanical method. How about a clay bar? Is a clay bar actually abrasive? Sure it is, but it's not substantially abrasive on it's own. I would caution against using it for the explicit purpose of wax removal. What it is very good at the shearing the surface. Anything sticking up beyond the normal plane of the car will be picked up by the clay. Think of it like shoveling snow. Your shovel will pick up the snow and if there is a rock sticking up from the pavement it will probably catch that and bring it with. In the case of the shovel, it's carrying the rock above the ground surface. In the case of clay, it's absorbed into the clay and now being rubbed against the paint, this makes the clay more abrasive. Still thinking about shoveling snow, what happens when we reach a sheet of ice on the surface? Do we pick it all up? Rarely, more often we pick up a portion and the shovel continues to glide over the ice scouring the top.
So how can we actually strip wax? Mechanically. If you are in a position where you need to be absolutely sure the wax is gone, it should be polished off. A very mild abrasive polish will be capable of removing the layer of wax and also refining the paint finish and removing ingrained dirt in the paint finish. This is a time consuming step compared to everything mentioned above, but it is the method to ensure removal.